The vacuum aluminizing chamber
by Dominic-Luc Webb

1. Introduction
I'll develop this page as the aluminizer develops. Please see links below to parts I
have made or currently working on.
The mission here is to aluminize a glass surface. I have been exploring how this
might be done at moderate to low cost for amateur astronomers. I also have had
in my mind for a considerable time to try other coatings, including filters. I
found a very low cost approach indeed. The starting material was a discarded
university ultracentrifuge, which was collected at no charge. The motor died,
leaving the vacuum fully intact, pulling a 10e-8 Torr or lower pressure within
a few minutes. I was already off to a great start. I could even have charged a
little money to haul the beast away. The chamber was large enough as is to
accommodate a 310 mm mirror. I spent much time reading about vacuum technique,
but having already acquired a working pump with chamber, the first "hands-on"
order of business for me was to understand the evaporation process and get
electrodes into the present chamber and build a larger chamber altogether later
as I get experience. The conventional way to aluminize uses a tungsten electrode
inside the chamber that heats a very small amount of aluminum sufficiently to
vaporize it, some of this evaporant ending up on the glass. To design this
beast, I first examined the physical properties of this system.
Physical properties of tungsten and aluminum and the vacuum procedure
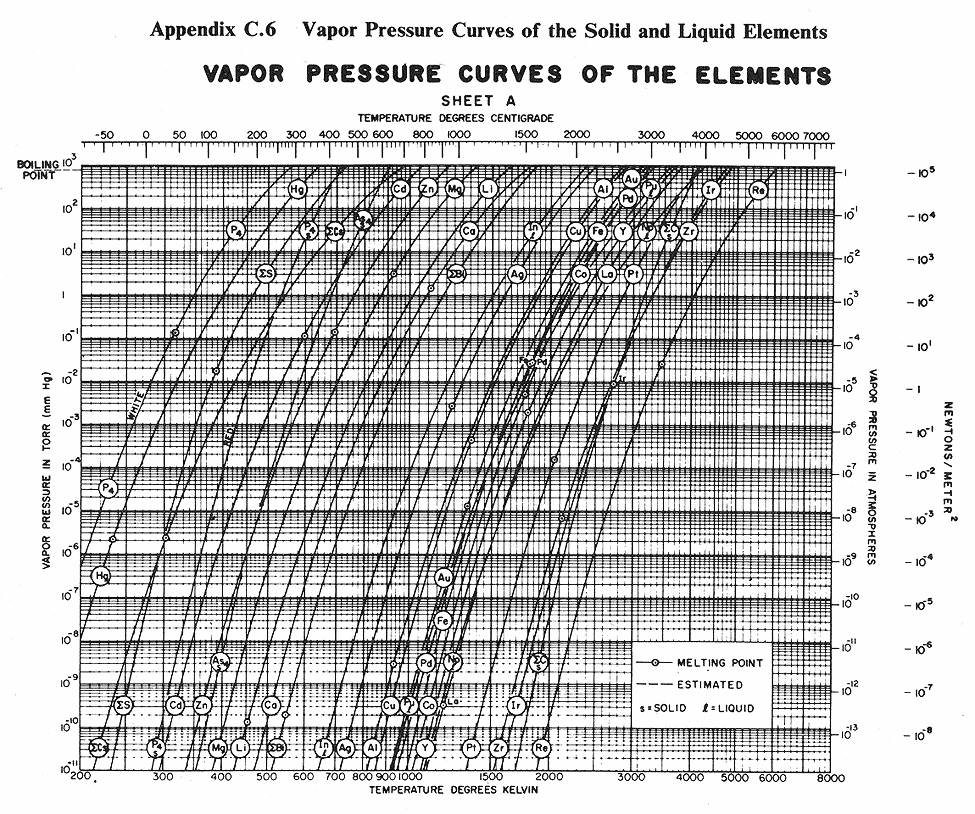
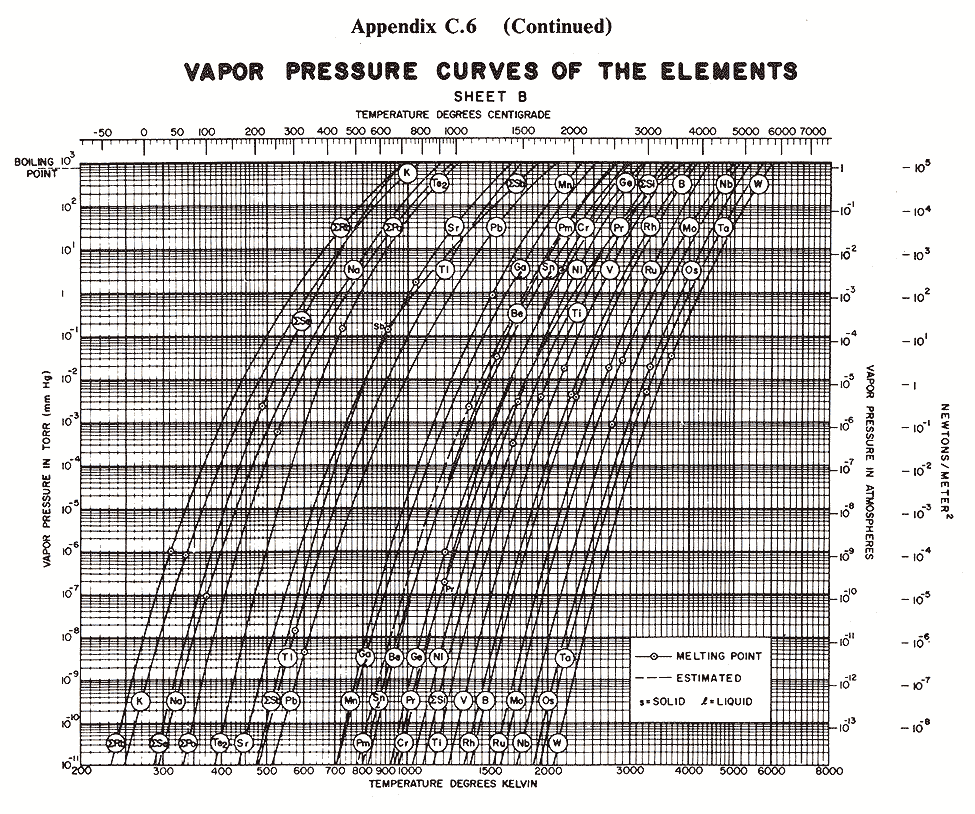
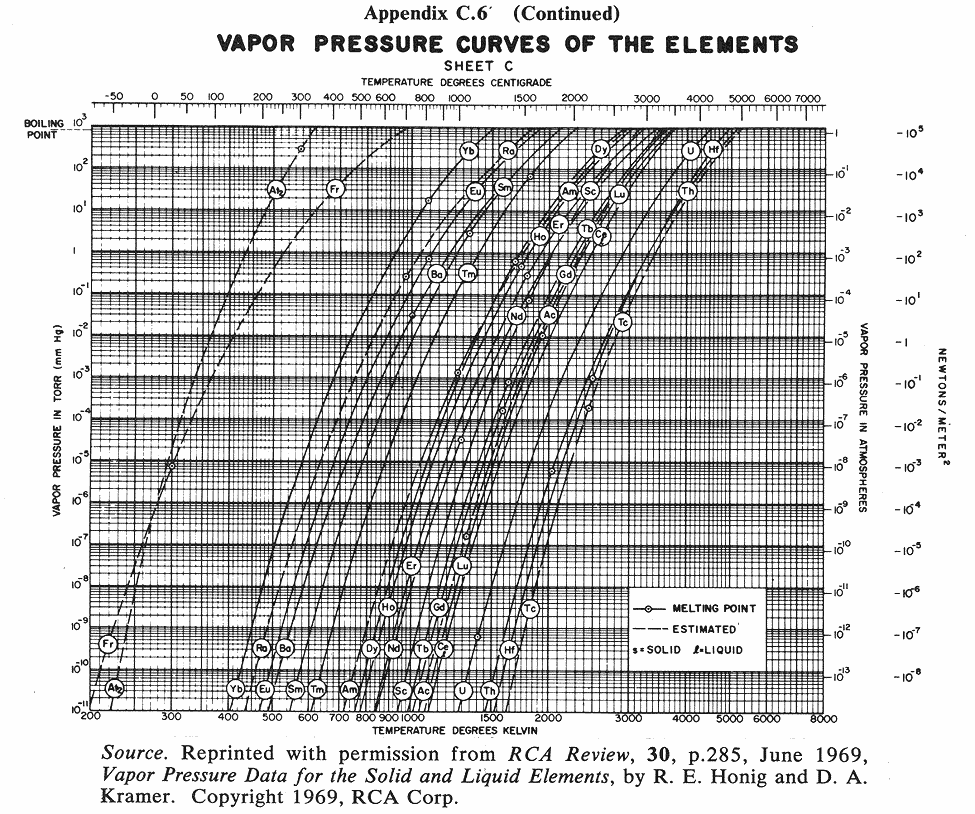
Below are some vapor pressure curves, which are very telling about aluminum,
tungsten and some other metals of interest in vacuum coating technique. They
are taken from (ref 1), kindly scanned by Kevin Anetsberger at Midwest Tungsten, a
source of low cost tungsten filaments (ref 3). Most noteworthy is that until one
reaches ridiculously low pressures that cannot be attained by the kind of homemade
aluminizers amateur astronomers are using, tungsten does not reach the vapor point
below 1900 C. At any of the pressures of interest in aluminizing (10e-4 to 10e-8 Torr),
aluminum can be vaporized at around 1000 C below the temperature at which tungsten
sublimes. Sublimation could predictably lead to rapid failure of the electrode,
limiting its lifespan. I estimate that between 1100 and 1300 C would be ideal for
vaporizing aluminum, although I still lack information about solubility of tungsten
in molten aluminum.
With the above in mind, Wien's Displacement Law can be considered.
The part in all textbooks and reference books: lambda = k / T,
where lambda is wavelength in meters and T is temperature in Kelvin.
The part often left out of most books; k = 2.893e-3 (units are
Kelvin*meters). I do not know why the value of k is left out, but it
can always be derived if one remembers that the Sun is 5500 K and
the peak wavelength is 530 nm, which incidentally, is the peak
senstivity of the human eye and also defintion of green in the RGB
color system. This is easy to remember. Consider the following table:
Substance Condition T (K) Lambda (nm)
+
Al melt 933 3101
Al *vapor 1300 2225
W **sublim. 2400 1205
* 10e-4 Torr, stated temp works at or below this pressure
** 10-8 Torr, pressures above this cannot sublime at stated temp
The human eye cannot see beyond about 750 nm, this being deep red.
The calculate wavelengths above are all well into the microns. This
tells us something useful.If the filament even attains a visible
deep red color, it is already hot enough to vaporize aluminum and
by the time there is enough of the black body curve to give an orange
color, tungsten is getting in the range it could sublimate. There is
a gap of around 1000 K between the point at which Al vaporizes and
at which tungsten sublimes. I surmise from these physical properties
that in actual use, the tungsten filament should probably not reach the
point where it turns orange and definitely yellow would be overkill.
Resistivity
Resistivity is calculated for 1 cm^2 at 25 degrees C. Here are some relevant resistivity
values (Ohm*cm):
W 5.51e-6
Al 2.828e-6
Fe 10e-6
Cu 1.724e-6
Nichrome 150e-6
References and Links (No space for PDFs, so use ref. 28 for articles):
Dominic-Luc Webb dlwebb@canit.se
http://www.megspace.com/science/stp/alum/alum.html



